Imagine a school with no teachers, a car with no engine, or a kitchen with no chefs. That’s what your cells would be like without enzymes. Life depends on millions of microscopic chemical reactions happening rapidly and reliably. But here’s the twist: these reactions don’t just happen—they’re controlled. They’re managed. They’re catalysed by proteins called enzymes. Understanding how enzymes work isn’t just key to acing your IB Biology exam—it’s the key to understanding how life operates on a molecular level.
Let’s break it down in the most IB-friendly, brain-friendly way possible.
🧬 What Is Metabolism? (C1.1.2)
Metabolism is the sum total of all chemical reactions happening in an organism. These reactions allow organisms to:
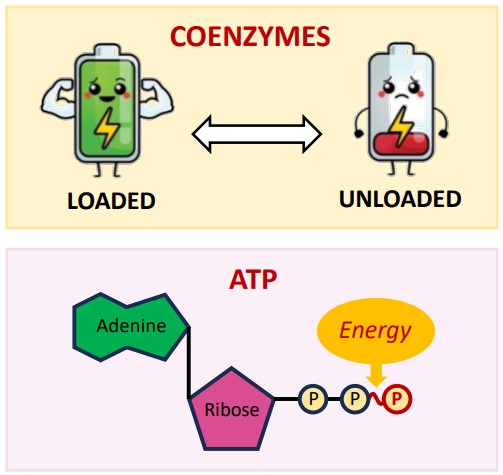
Obtain and use energy (like producing ATP)
Build complex molecules from simpler ones
Break down large molecules to recycle or release energy
These reactions are classified into two types:
| Reaction Type | Description | Example | Water Used or Released? |
|---|---|---|---|
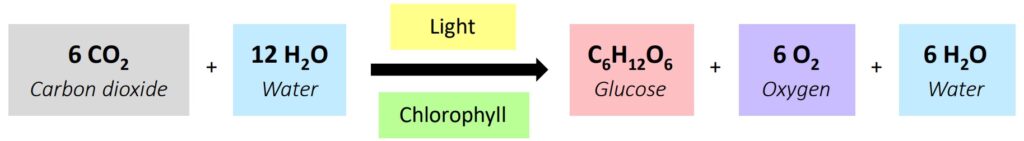
Anabolic 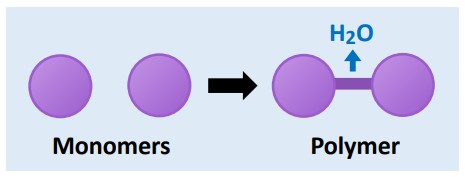 | Builds complex molecules from simpler ones (constructive metabolism) | Photosynthesis, protein synthesis | Water released (condensation) |
Catabolic 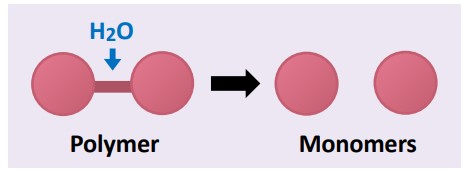 | Breaks complex molecules into simpler ones (destructive metabolism) | Cellular respiration, digestion | Water consumed (hydrolysis) |
💡 Link to IB: This directly aligns with C1.1.3, understanding anabolic vs. catabolic reactions.
⚙️ Enzymes: Biological Catalysts (C1.1.1, C1.1.2)
What Are Enzymes?
Enzymes are globular proteins that function as biological catalysts.
They speed up chemical reactions by lowering the activation energy needed.
Importantly, enzymes are not consumed or permanently changed by the reactions they catalyse.
Key Properties:
Reusable: They can be used repeatedly.
Substrate-specific: Each enzyme works on a specific molecule (its substrate).
Efficient: Can increase reaction rates by over a million times.
🧪 Example: Lipase breaks down lipids; amylase breaks down starch; DNA polymerase builds DNA strands.
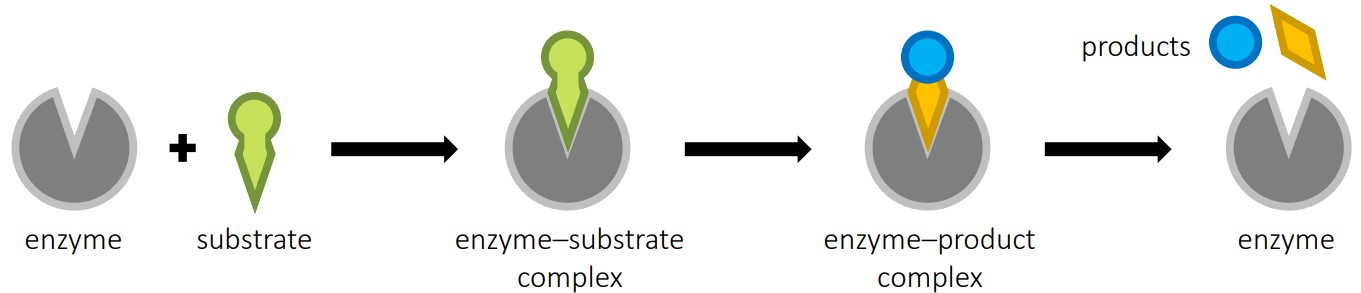
🔒 Active Site & Enzyme Specificity (C1.1.4, C1.1.5, C1.1.7)
What Is the Active Site?
The active site is a special groove or pocket on the enzyme where the substrate binds.
Its shape and chemical environment match the substrate.
Two Models of Binding:
| Model | Description | Analogy |
|---|---|---|
| Lock & Key | Enzyme and substrate fit perfectly | Like a house key fitting a lock |
| Induced Fit | Enzyme changes shape slightly to fit the substrate | Like a glove adjusting to your hand |
🧠 IB Tip: Induced fit is the more accepted model because it explains enzyme flexibility and reactivity (C1.1.5).
🌡️ How Do Enzymes Work? (C1.1.6, C1.1.10)
Molecular Motion & Collisions
Enzymes and substrates move randomly in fluids (Brownian motion).
A reaction happens when a substrate collides with the active site in the correct orientation.
Increasing the kinetic energy (e.g., by heating) increases collision frequency.
Activation Energy
Activation energy is the minimum energy needed to start a reaction.
Enzymes lower this barrier, making it easier and faster for the reaction to proceed.
🎯 Analogy: Think of enzymes like tutors who make hard problems easier to solve by showing shortcuts—same result, just faster.
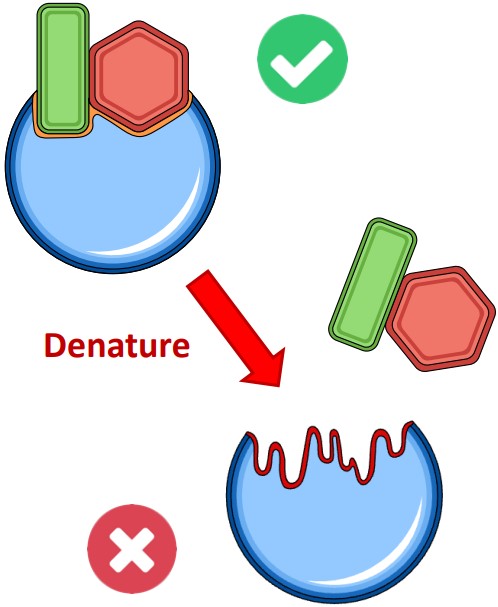
⚠️ Denaturation: When Enzymes Go Wrong (C1.1.7)
What Is Denaturation?
Denaturation is a loss of the enzyme’s 3D structure, especially the shape of the active site.
It happens when external conditions disrupt internal bonds (e.g., hydrogen bonds, ionic interactions).
Causes:
High temperature: Breaks bonds due to excessive kinetic energy.
pH changes: Alter charge distribution, affecting bonding and folding.
⚠️ Once denatured, most enzymes cannot regain function. A few may refold if returned to optimal conditions (reversible denaturation).
📊 Factors Affecting Enzyme Activity (C1.1.8)
1. Temperature
| Temperature | Effect on Enzyme |
|---|---|
| Low | Molecules move too slowly → few collisions |
| Optimal | Maximum activity due to frequent successful collisions |
| High | Bonds break → enzyme denatures → activity drops sharply |
🔥 Enzymes usually peak around 37°C in humans.
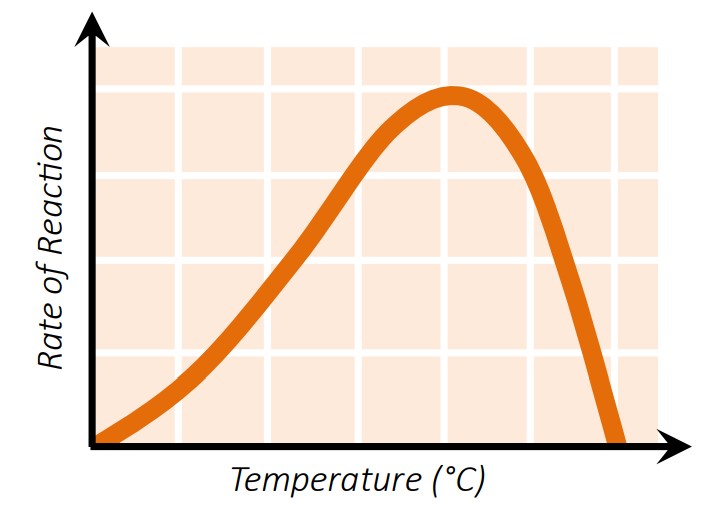
2. pH
Each enzyme has an optimal pH.
Deviating from this range changes the enzyme’s charge and shape, leading to denaturation.
🧪 Example: Pepsin works best in the stomach (~pH 2), while amylase prefers saliva (~pH 7).
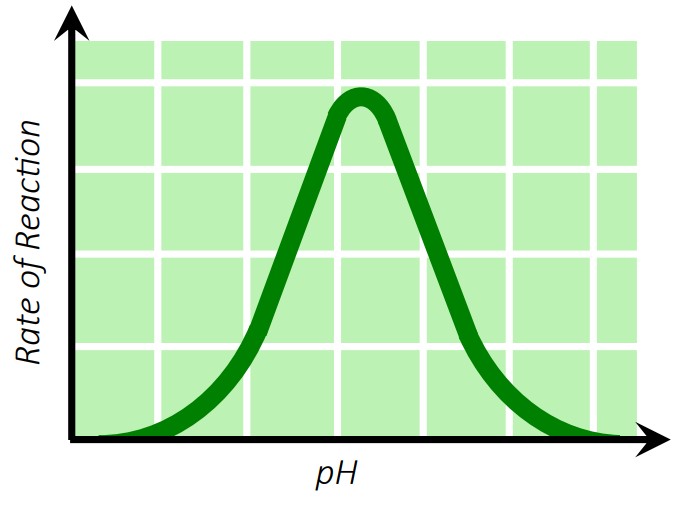
3. Substrate Concentration
More substrate = more collisions = increased reaction rate—up to a point.
Once all enzyme active sites are saturated, adding more substrate does not increase the rate.
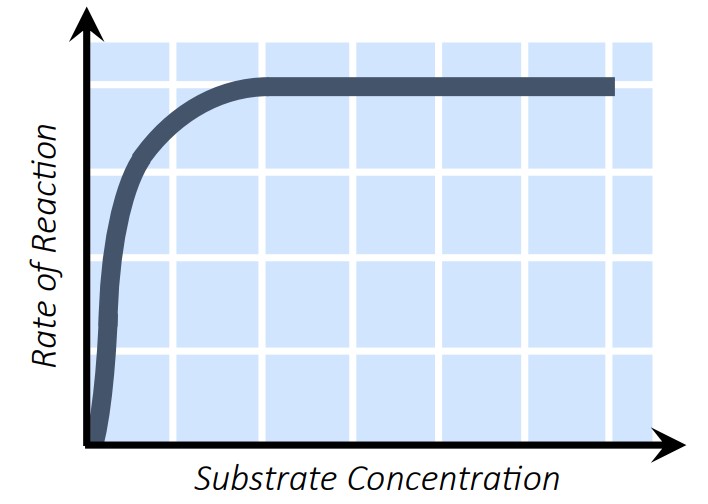
4. Enzyme Concentration
More enzyme = more available active sites = faster reactions (as long as there’s enough substrate).
In real-life cells, enzymes are usually in low concentrations because they’re reusable.
 Measuring Enzyme Activity (C1.1.9)
Measuring Enzyme Activity (C1.1.9)
How Do We Measure It?
Rate of product formation (e.g., how fast oxygen is released in a catalase reaction)
Rate of substrate disappearance (e.g., how fast starch disappears in an amylase reaction)
Units: typically in mol/min, μmol/sec, etc.
On a graph, enzyme activity often shows:
A rising phase (more collisions),
A peak (optimal conditions),
A decline (due to denaturation or saturation).
 Real IB Examples: Linking Metabolism to Key Processes
Real IB Examples: Linking Metabolism to Key Processes
| Process | Type | IB Context |
|---|---|---|
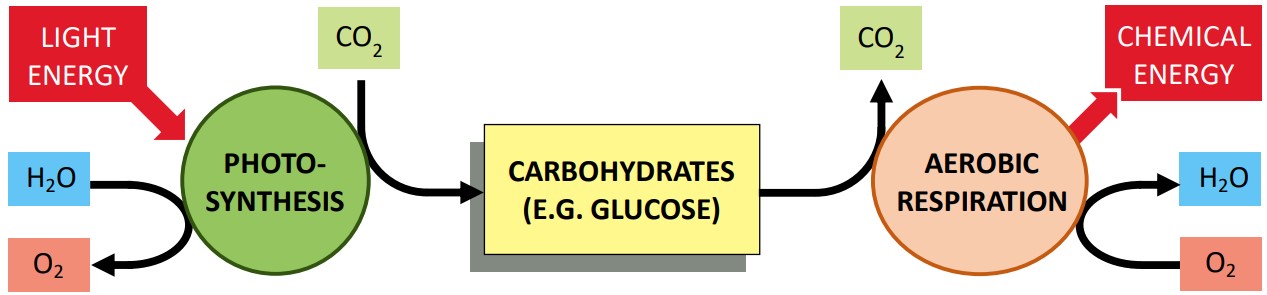
| Photosynthesis | Anabolic | Builds glucose from CO₂ and H₂O (uses light energy) |
| Cellular Respiration | Catabolic | Breaks down glucose to release ATP (exergonic) |
| Protein Synthesis | Anabolic | Joins amino acids via condensation |
| Digestion | Catabolic | Hydrolyses macromolecules to monomers |
 Key Takeaways for IB Success
Key Takeaways for IB Success
Enzymes control metabolism by lowering activation energy, making life’s reactions possible at biological temperatures.
Structure determines function: The enzyme’s 3D shape is critical for its specificity and efficiency.
Environmental factors affect enzyme function: Too hot, too acidic/basic, or too crowded? The enzyme might fail.
Use visuals: Draw diagrams of enzyme-substrate interactions, energy profiles, and activity curves. They help solidify understanding.
 Bonus Tip: Vocabulary for the IB Exam
Bonus Tip: Vocabulary for the IB Exam
Catalyst: Substance that speeds up a chemical reaction without being used up
Active Site: Region of an enzyme that binds the substrate
Substrate: The reactant that an enzyme acts on
Denaturation: Structural change that results in loss of biological activity
Metabolism: All chemical reactions in an organism
 FAQ: Enzymes & Metabolism – IB Clarified
FAQ: Enzymes & Metabolism – IB Clarified
All enzymes are catalysts, but not all catalysts are enzymes. Enzymes are biological (made of proteins); chemical catalysts can be inorganic.
Enzyme active sites are specific in shape and chemistry—like a glove fits only certain hands. That’s why enzymes don’t work on everything.
Yes, if the reaction is reversible and the product can also bind to the active site, enzymes may catalyse reverse reactions under the right conditions.
Most are synthesised and directed to specific cellular locations by signal sequences in their amino acid chains or are anchored in membranes.
Not always. Some enzymes can refold if mild conditions are restored, but most undergo irreversible denaturation due to lost structural integrity.

 Measuring Enzyme Activity (C1.1.9)
Measuring Enzyme Activity (C1.1.9) On a graph, enzyme activity often shows:
On a graph, enzyme activity often shows: Real IB Examples: Linking Metabolism to Key Processes
Real IB Examples: Linking Metabolism to Key Processes Key Takeaways for IB Success
Key Takeaways for IB Success Bonus Tip: Vocabulary for the IB Exam
Bonus Tip: Vocabulary for the IB Exam FAQ: Enzymes & Metabolism – IB Clarified
FAQ: Enzymes & Metabolism – IB Clarified